Drug Delivery Device Implanter
Summary
The client needed a reliable insertion and removal solution for an osmotic pump-based drug delivery system that provided six-month dosing for diabetes patients. We developed a guided cannula system for precise depth control and a minimalistic wireform removal tool, ensuring accurate placement, ease of use, and cost-effective, environmentally conscious design.
Problem
The client had developed an innovative drug delivery system for diabetes treatment, designed to provide patients with medication at six-month intervals. This system utilized an embedded capsule that gradually dispensed the drug through an osmotic pump, eliminating the need for frequent dosing.
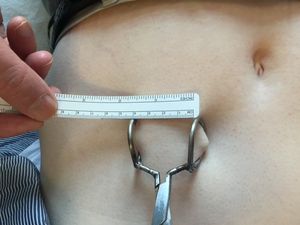
While the client had already developed an insertion tool, they encountered a critical challenge: ensuring the capsule could be placed at the correct depth with precision and ease. The existing tool lacked a reliable depth control mechanism, making it difficult for physicians to insert the capsule accurately. Additionally, there was no standardized method for removing and replacing the capsule once it had been depleted. The client sought a solution that would enable seamless implantation at the appropriate depth while also facilitating efficient and minimally invasive removal when replacement was needed.
Solution
A key challenge in the insertion process was that the cannula’s leading edge served as the cutting surface. As the device was inserted, it needed to be rotated to allow the cannula to create a tunnel just beneath the skin. This requirement meant that any guiding mechanism needed to accommodate rotational movement while still ensuring precise depth control.
To address this, we explored multiple design approaches for controlling the cannula’s insertion depth. After testing various concepts, the most effective solution proved to be a pre-attached guide that was integrated with the cannula. This guide featured a slight angular offset in the through-hole, allowing the leading edge of the cannula to cut smoothly while preventing soft tissue from being pinched between the guide and the cannula. This design ensured a controlled and accurate insertion process, reducing variability in placement and improving ease of use for physicians.
We went through multiple iterations of the design, fabricating and testing prototypes using silicone-based training models. Once the performance met our expectations, we conducted further testing in cadaver labs to validate its effectiveness under realistic conditions. After refining the design based on these trials, we produced molded components for evaluation in volunteer studies. These real-world tests confirmed the reliability, safety, and usability of the final design. Additionally we designed a version where the cannula would retract into the handle after pump deployment to reduce the risk of needle-stick injuries.
For the capsule removal process, we developed a simple yet effective wireform tool that could hook securely under the implanted device. This allowed the physician to lift the capsule while using a scalpel to open one end of the tunnel, enabling smooth extraction with minimal tissue disruption. Our primary objectives for this removal tool were to ensure ease of use, hands-free operation, and cost-effectiveness. Given that the device was designed for single-use, we prioritized minimizing plastic consumption to reduce environmental impact while maintaining structural integrity.
By integrating precise insertion guidance and a simplified removal mechanism, we delivered a comprehensive solution that enhanced both the implantation and replacement processes. The final design optimized efficiency, reduced procedural complexity, and ensured a high degree of reliability for physicians and patients alike.
Images